While you’re relaxing at a nail salon and your nail technician applies gel polish on your fingertips, you might not realize that complicated chemistry is taking place. Gel nails were first introduced in the U.S. nail industry in the early 1980s but were initially met with confusion and sub-par results. Gel nails only became popular in the ‘90s and have risen in popularity since.
A nail salon customer might first see the nail technician apply a layer of gel on the nails. They then turn on a UV or LED lamp, place your hands with the still-wet gel under the lamp and ask you to wait. After around two minutes with a UV lamp or a minute with an LED lamp, your hands come out with a beautiful manicure. But the seemingly simple – and eye-catching – product is full of complicated chemicals and processes. According to NAILS Magazine, gel nails and acrylic nails are similar in that they are both actually acrylics, any synthetic resins or fibers that are based on derivatives of acrylic acid and methacrylic acid. These acids start off as monomers (building blocks of larger science stuff called polymers) and are quite viscous, just like regular nail polish. They undergo a process called polymerization as they link up and become a solid.
Polymerization, according to NU chemistry Professor SonBinh Nguyen, is a process where individual molecules, called monomers, open up and connect to one another.
“Monomers are saturated and cannot bond to other monomers,” Nguyen said. “When you add a catalyst, it acts as a plier and opens up one end of the monomer and allows it to connect to a second monomer.”
The process, according to Nguyen, is complicated. In order to demonstrate polymerization, he used the polymerization of the styrene monomer as an example. Take a look at this illustration.
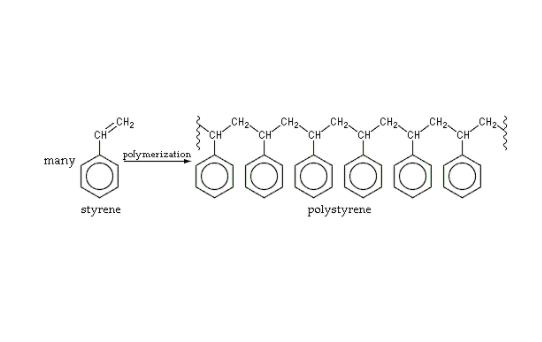
The styrene monomer on the left is stable. It has a double bond between the CH and the CH2, as represented by the two dashed lines. Saturation, Nguyen explains, is when there is a double bond that closes the molecule into itself. When this bond is broken, monomers can then be connected with a catalyst, which is something that initiates a reaction. Some polymerization processes do not need a catalyst, but a catalyst is needed for both styrene and gel polish, albeit in different forms. When the double bond is broken, the CH2 will need another CH to bond to in order to be stable. Thus, a CH2 of one styrene monomer will connect to a CH of another monomer with a single bond. This will create one bigger polymer called a polystyrene. This whole process is polymerization, and it can transform the physical characteristic of the substance.
Gel nail polish, however is quite different and more complicated than styrene, according to Nguyen.
“Nail polish is different [from simple polymerization],” Nguyen said. “Nail polish gel don’t use small molecules, they are much bigger and are thus viscous. The UV light acts as the catalyst and provides the energy to open up the molecule.”
In gel nails, there are chemicals called photoinitiators, according to NAILS Magazine. Photoinitiators absorb light and convert it into energy that is needed for the polymerization process. Photoinitiators only react to lights that have a certain wavelength. Most photoinitiators found in gel nails react to wavelengths found in the UV range, from roughly 100 nm. to 400 nm. – the same wavelengths provided by UV lamps or LED lamps you find at a nail salon. UV light therefore acts as the catalyst in the polymerization process in gel nails, transforming a semi-liquid substance into a solid manicure. This is quite different from acrylic nail polish, which is a two-step process that requires the a catalyst to be introduced to a monomer.
Scientists have explored polymerization since the early 20th century – yet, only recently has this technology been applied for a practical use. Years of development in research labs have made it possible for clients to have perfect, long-lasting manicures. Next time you go to a nail salon, take some time to appreciate the technology that is happening right at your fingertips.